Proteinstability
The precise characterization of protein solutions is essential for optimal process design and formulation development. A critical parameter in the development of biopharmaceuticals is the solubility of the molecule during the process and the long-term stability of the pharmaceutical formulation. Therefore, our research group is intensively engaged in the investigation of protein-protein interactions, the resulting phase behavior, the predictability of the phase behavior and its manipulation. We investigate model proteins, pharmaceutically relevant molecules such as antibodies, different classes of enzymes, as well as viruses and virus-like molecules in ideally diluted solution and in highly concentrated formulations.
Literature
Protein Phase Behavior
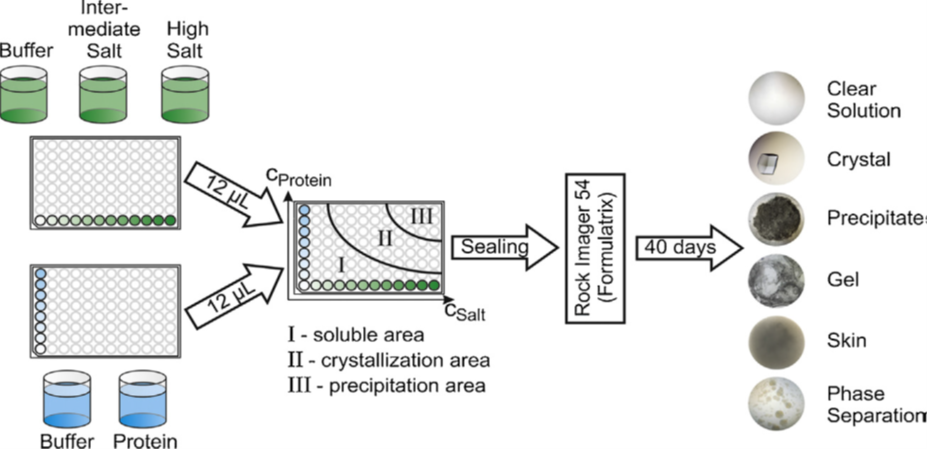
For the pharmaceutical industry, the stability of biologically active components during processing, formulation and storage is of crucial importance in order to ensure patient safety and product activity. An important stability criterion is the phase behavior of the target molecule. It can be distinguished between the soluble, crystalline and precipitated phase state as well as gel formation. Controlled crystallization and precipitation offer the biopharmaceutical industry a cost effective alternative to conventional separation methods and are acknowledged for formulation purposes. However, undesired aggregation may lead to product loss and changes in the three-dimensional structure of the target molecule. Therefore, knowledge of protein phase behavior is essential for downstream process design.
At our institute, a screening method to generate phase diagrams in high throughput on an automated liquid handling station in microbatch scale was developed. Using a high resolution camera, the generated phase diagrams are evaluated regarding their phase behavior. Using this method, the influence of various process parameters, such as type and concentration of salts or polymers, pH or temperature for various biologically active components can be examined.